It's easy to see why the modern lighting industry is obsessed with these little devices. With a fraction of the energy used by conventional light bulbs, you get much more light output with significantly less heat.
Oversimplified: LEDs work by allowing electricity to flow through a metal that has gone through a process to remove its charge. As the electricity recharges the metal, it lights up due to a phenominon known as Electroluminescence. One of the most common metals is zinc-sulphide, which when doped with copper will produce green light, or with silver will produce blue light.
With different materials and manufacturing processes, we are able to make LEDs that produce any colour through the visible spectrum, and beyond. The fact that LEDs can be turned on or off instantly and with great accuracy means that infrared LEDs power most TV remotes. LEDs can also be made to give of ultraviolet light, perfect for glow-in-the-dark lighting!


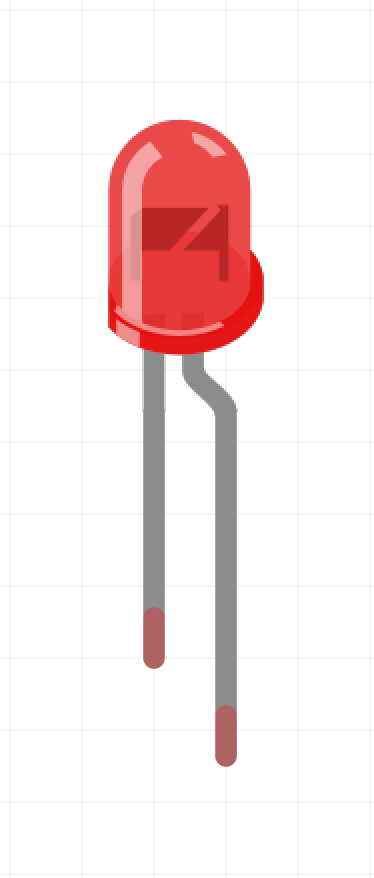
This is what an LED looks like on an electrical schematic and in real life. Notice that the LED has two legs. The longer one is the annode the shorter is the cathode.
Electricity must go from the annode to the cathode or else the LED will not work. If you LED does not work, try swapping the annode and cathode around, 9 times out of 10 that will fix it.
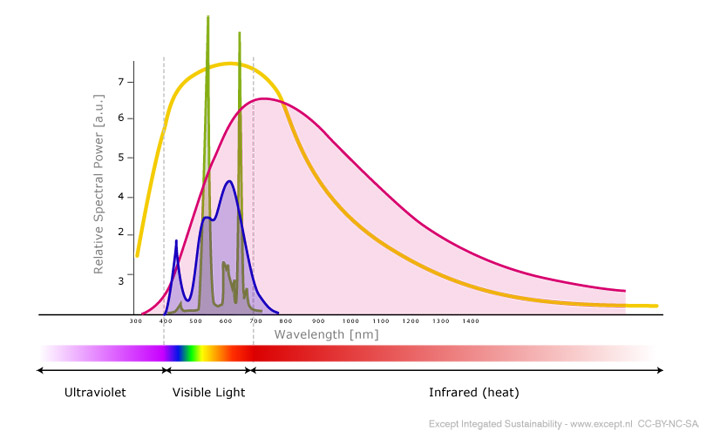
LEDs are capable of producing light starting in the ultraviolet spectrum, going right through the visible spectrum, and into the infrared spectrum. For our lab, we really only care about the visible light.
 An LED stagelight. If you look, there are some in the DARE Disctrict
An LED stagelight. If you look, there are some in the DARE Disctrict